This test evaluates the psychotic like effects of a pharmacological challenge (NMDA receptor antagonist, MK-801) by studying deficits in pre-pulse inhibition. This test is applicable to rats and mice.
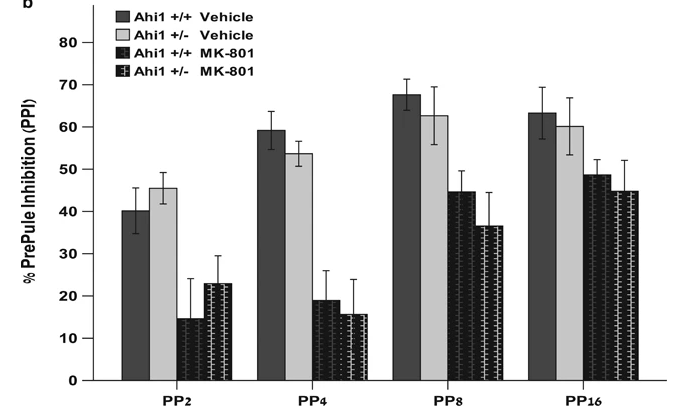
Mice from Ahi1+/+ and Ahi1+/- genotypes were injected i.p. with saline and allowed to acclimate to the apparatus (ventilated soundproof startle chambers, San Diego Instruments, containing a plexiglass cylinder to house the animal during the test). Thirty min later the test was started. Pulse alone trials (Pulse) consisted of a single white noise burst (110 dB, 40 ms). Prepulse–pulse trials (PP) consisted of a prepulse of an intensity of 2, 4, 8, or 16 dB above background noise (PP2, PP4, PP8, or PP16, respectively), followed 100 ms after prepulse onset by a startling pulse (110 dB, 40 ms). No-stimulus trials consisted of background noise only (68 dB). Sessions were structured as follows: 1) 5-min acclimation at background noise level; 2) ten pulse trials; 3) ten blocks each containing all six trials (Pulse, PP2, PP4, PP8, or PP16, No-stimulus) in pseudorandom order; 4) ten Pulse trials. The force intensity for each trial was recorded as the startle level. The percentage PPI induced by each prepulse intensity was calculated as [1 − (startle amplitude on prepulse trial) / (startle amplitude on pulse alone)] × 100. One week later, mice were injected i.p. with MK-801 0.15 mg/kg and then retested using the same procedure described above. Although prepulse intensity and treatment (vehicle vs MK-801) both had a significant main effect on %PPI (F2.119,38.143=34.942, P<0.0005 and F1,18=18.826, P<0.0005, respectively), main effect of genotype, genotype × prepulse intensity interaction, genotype × treatment interaction and genotype × prepulse intensity × treatment interaction were all nonsignificant. n=9–11 mice per genotype. Reproduced from Lotan et al, Molecular Psychiatry, 2014.
References:
Lotan A, Lifschytz T, Slonimsky A, Broner EC, Greenbaum L, Abedat S, Fellig Y, Cohen H, Lory O, Goelman G, Lerer B. Neural mechanisms underlying stress resilience in Ahi1 knockout mice: relevance to neuropsychiatric disorders. Mol Psychiatry. 2014 Feb;19(2):243-52. doi: 10.1038/mp.2013.123. Epub 2013 Sep 17.
van den Buuse M. Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophr Bull. 2010.
